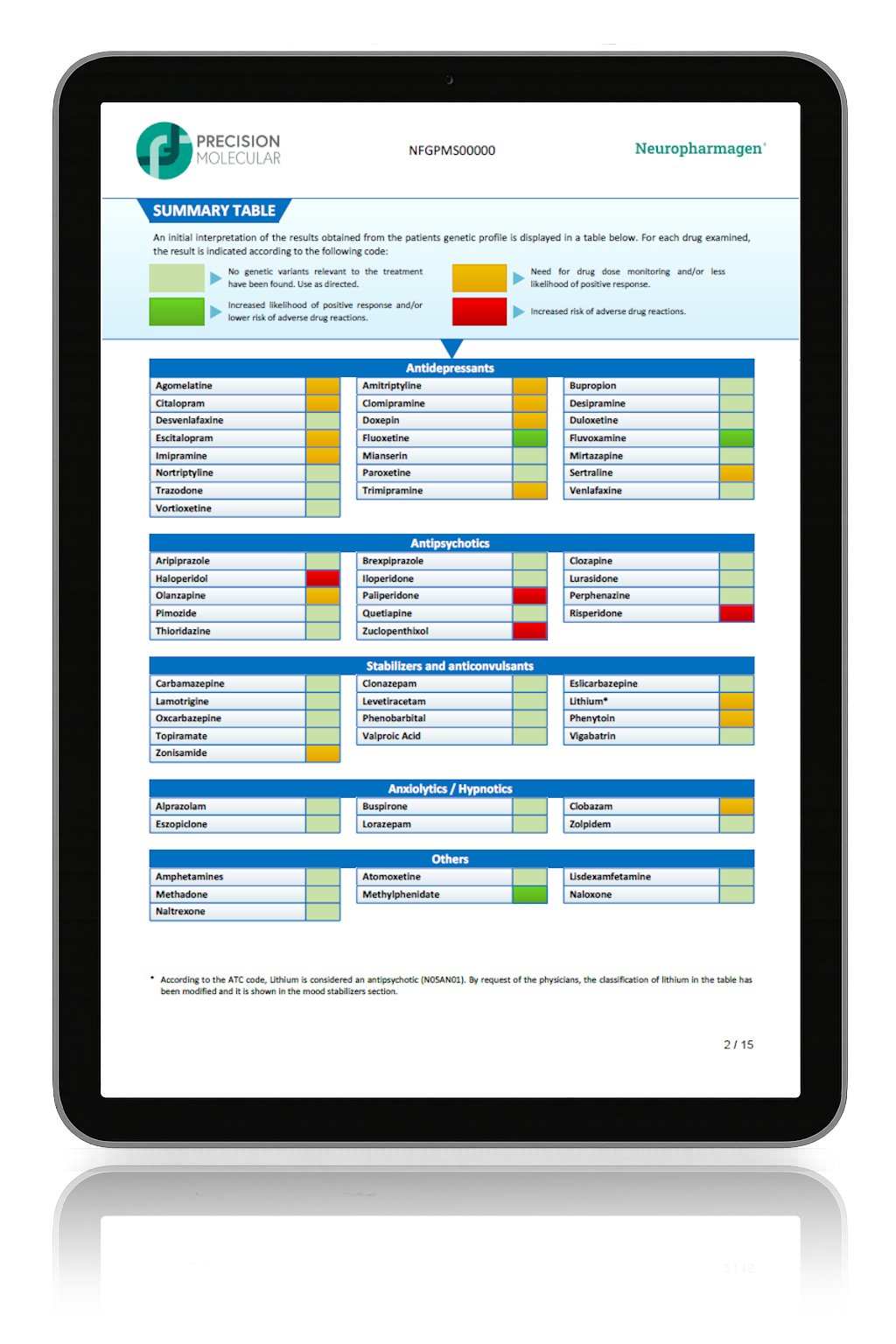
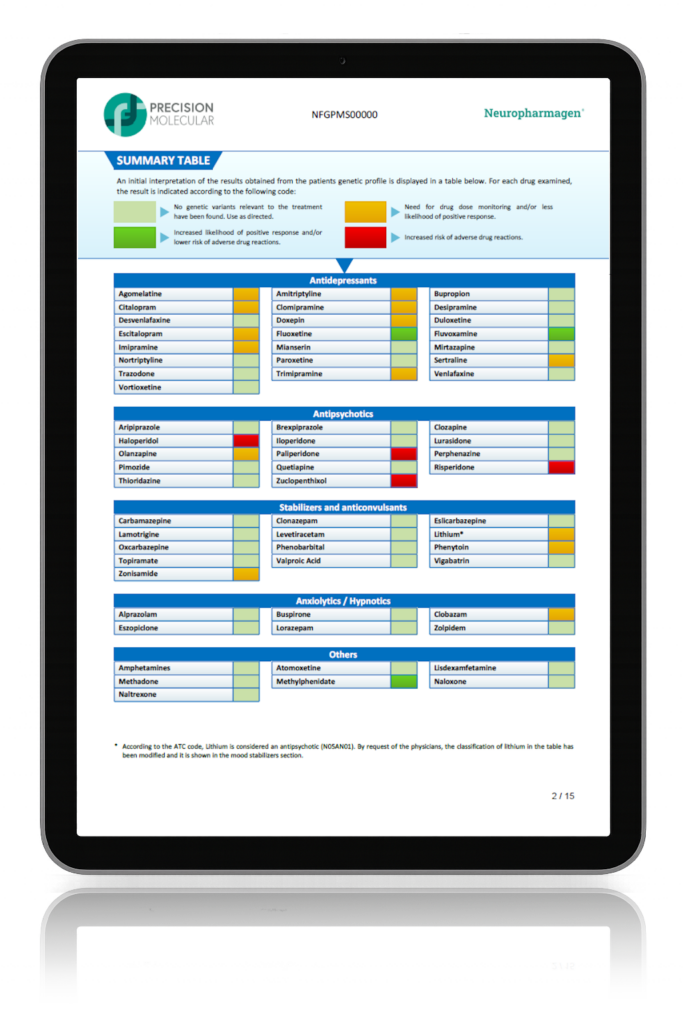
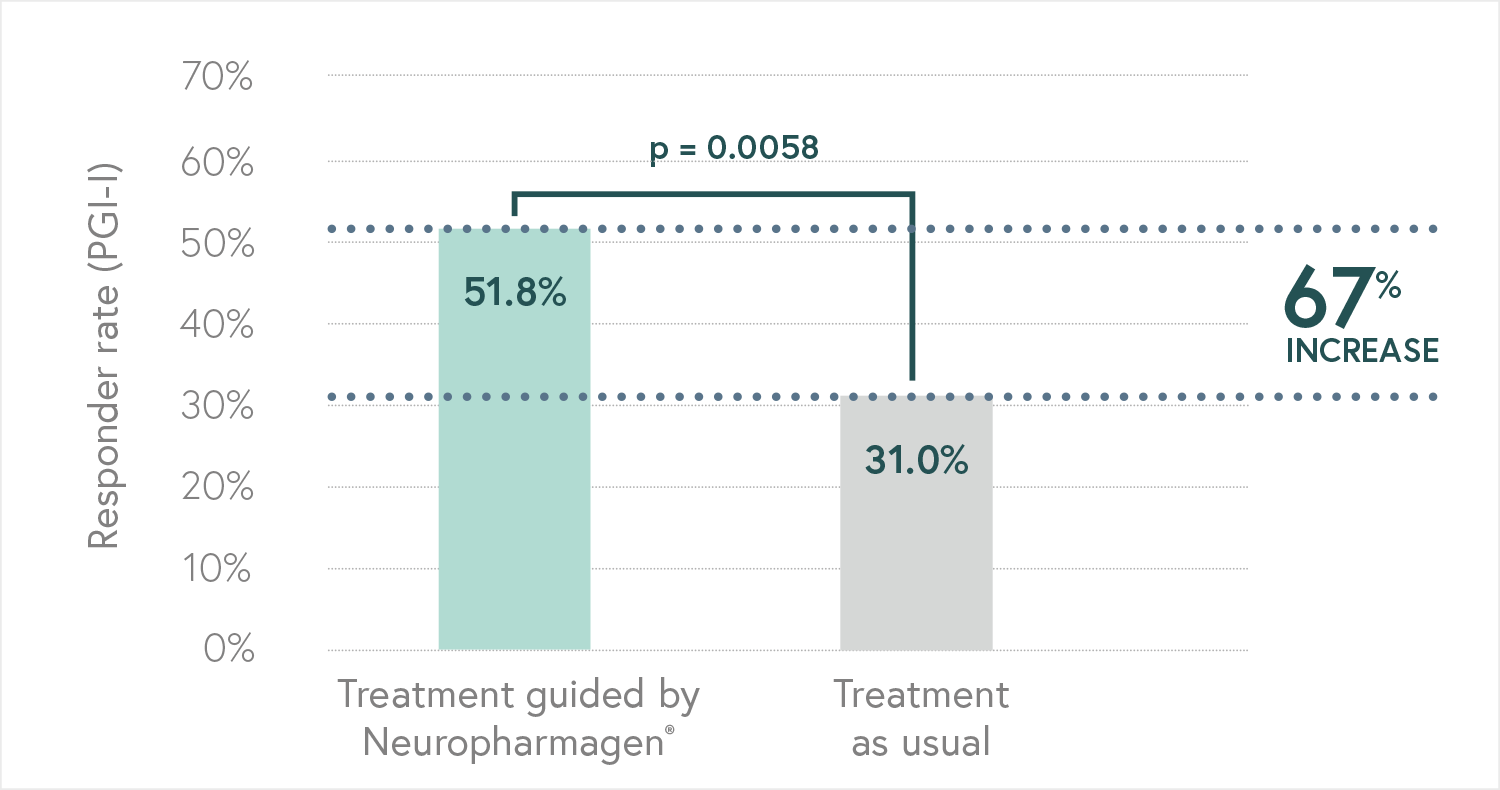
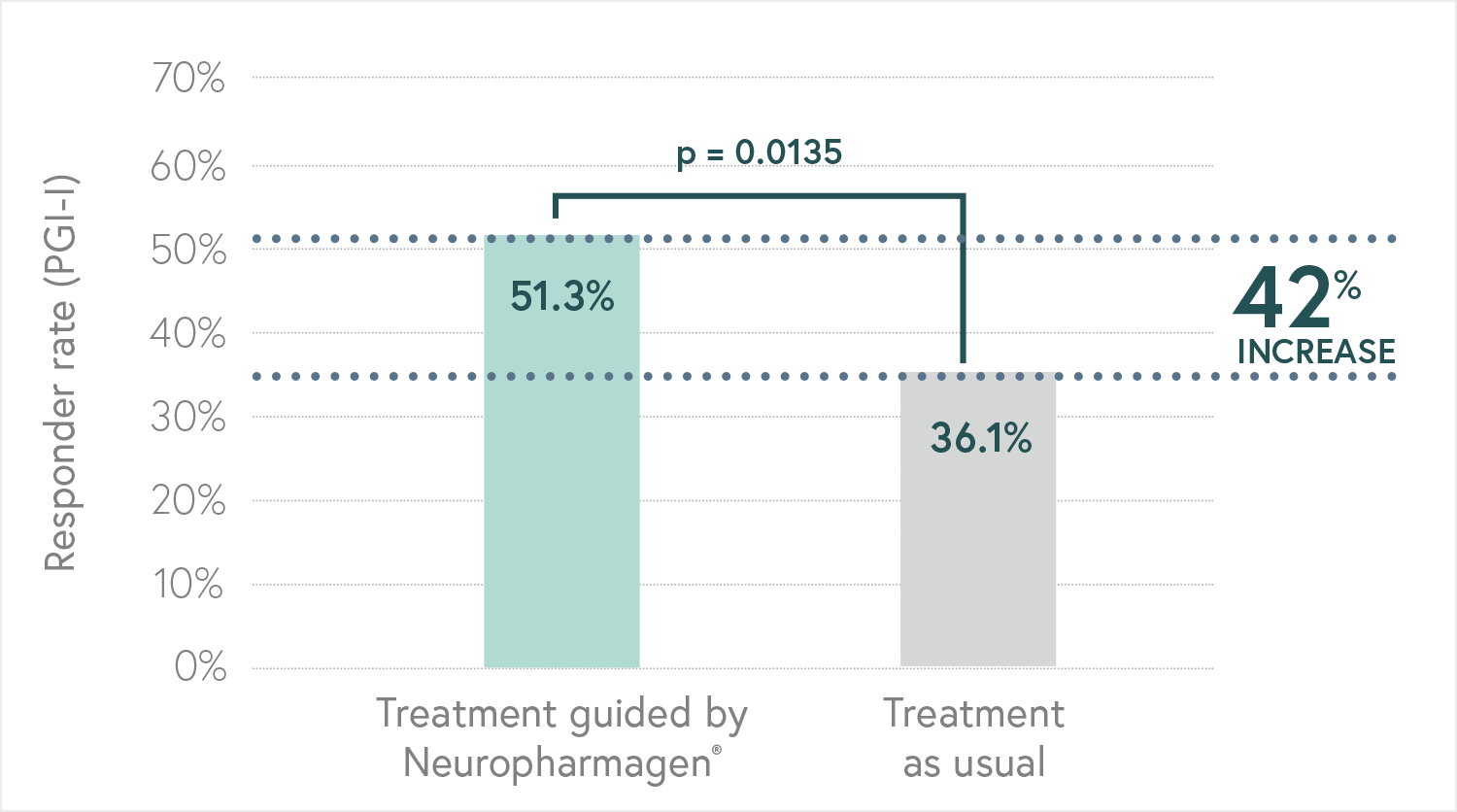
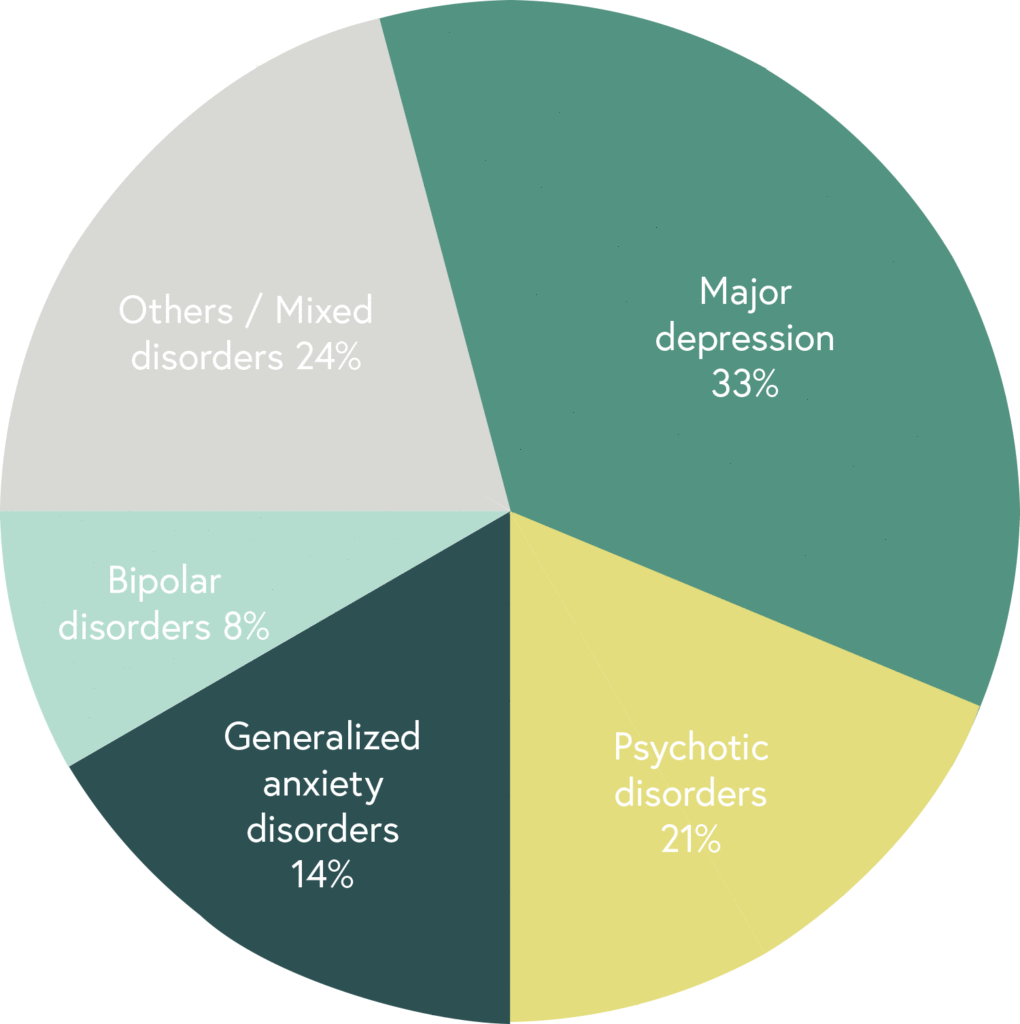
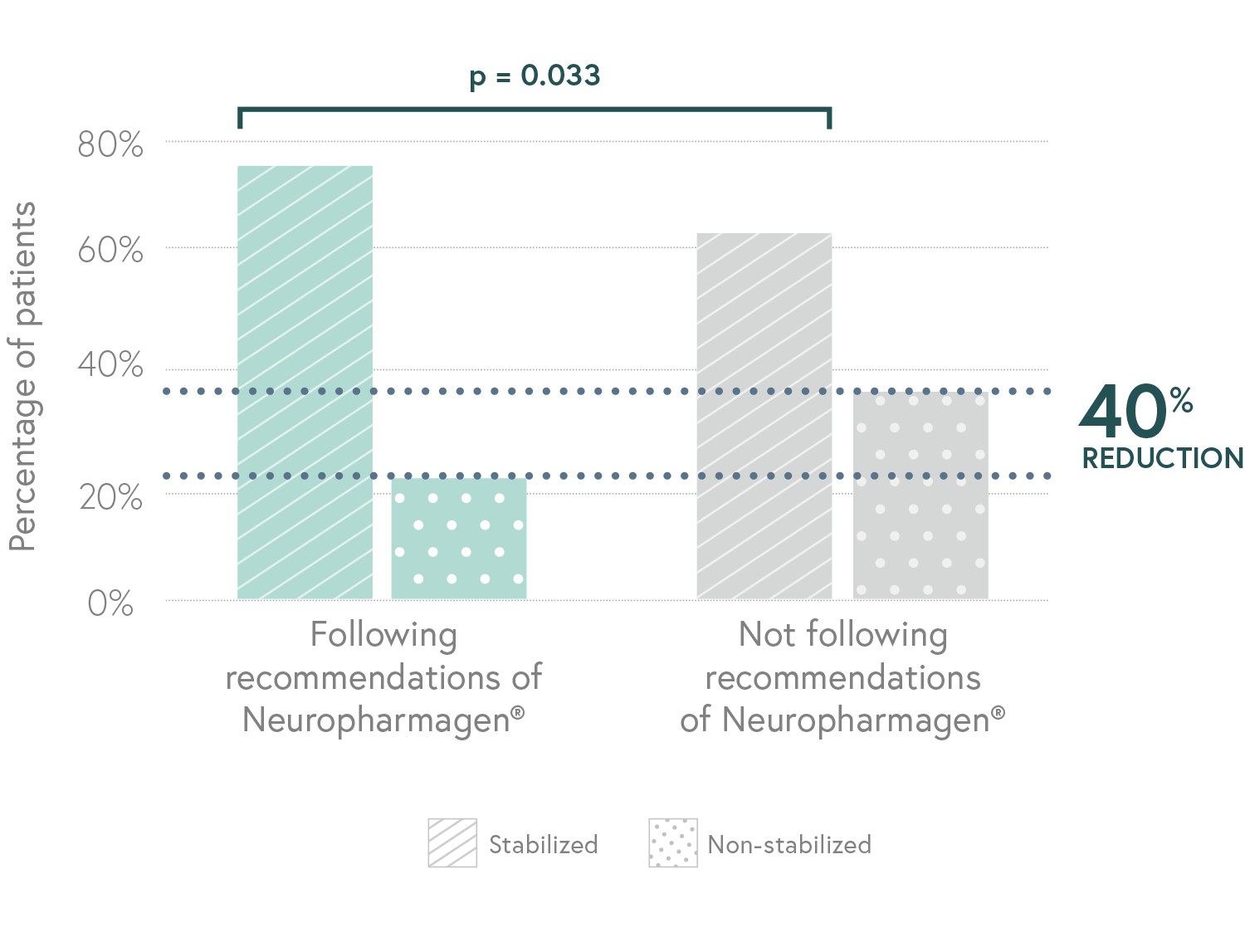
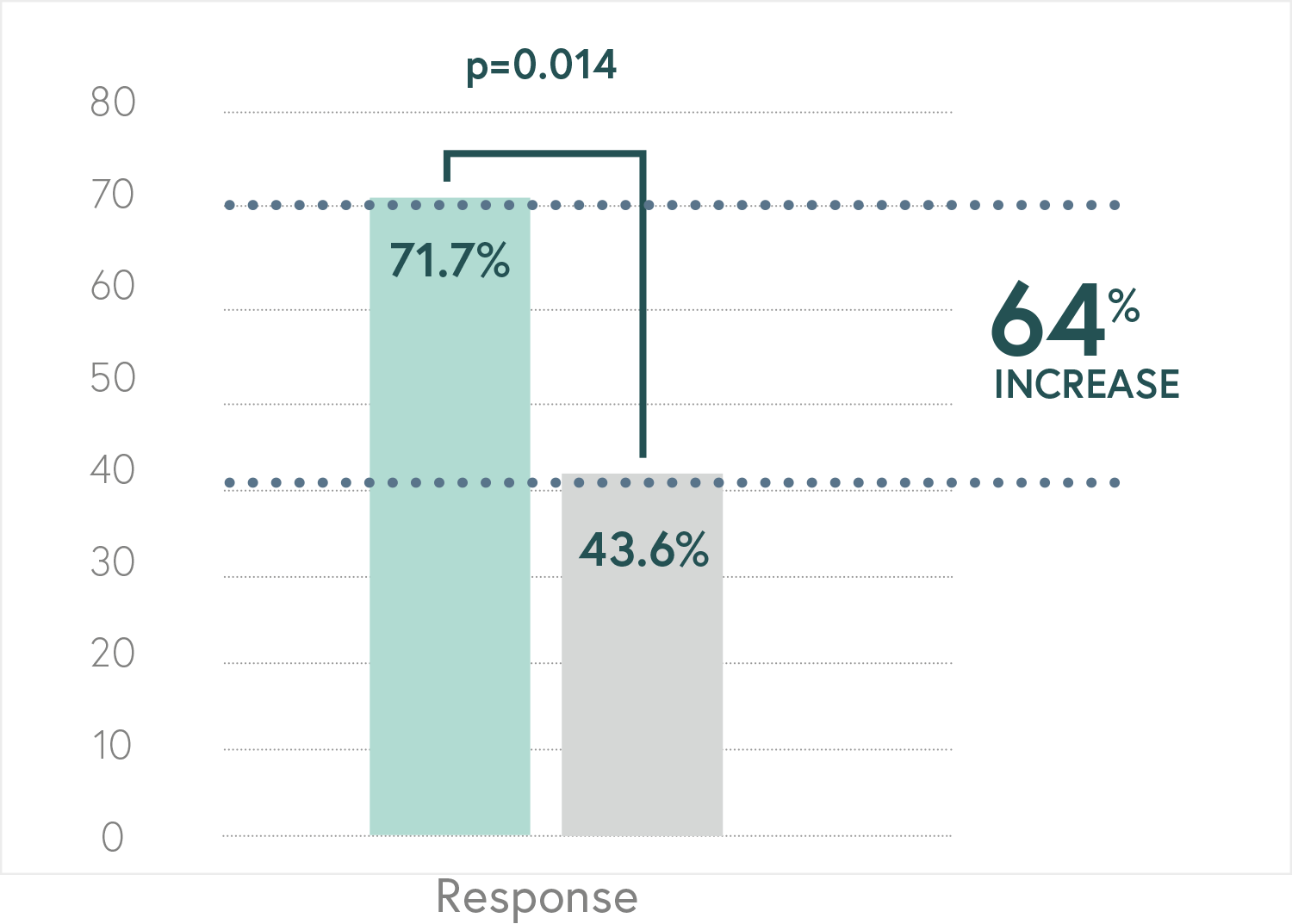
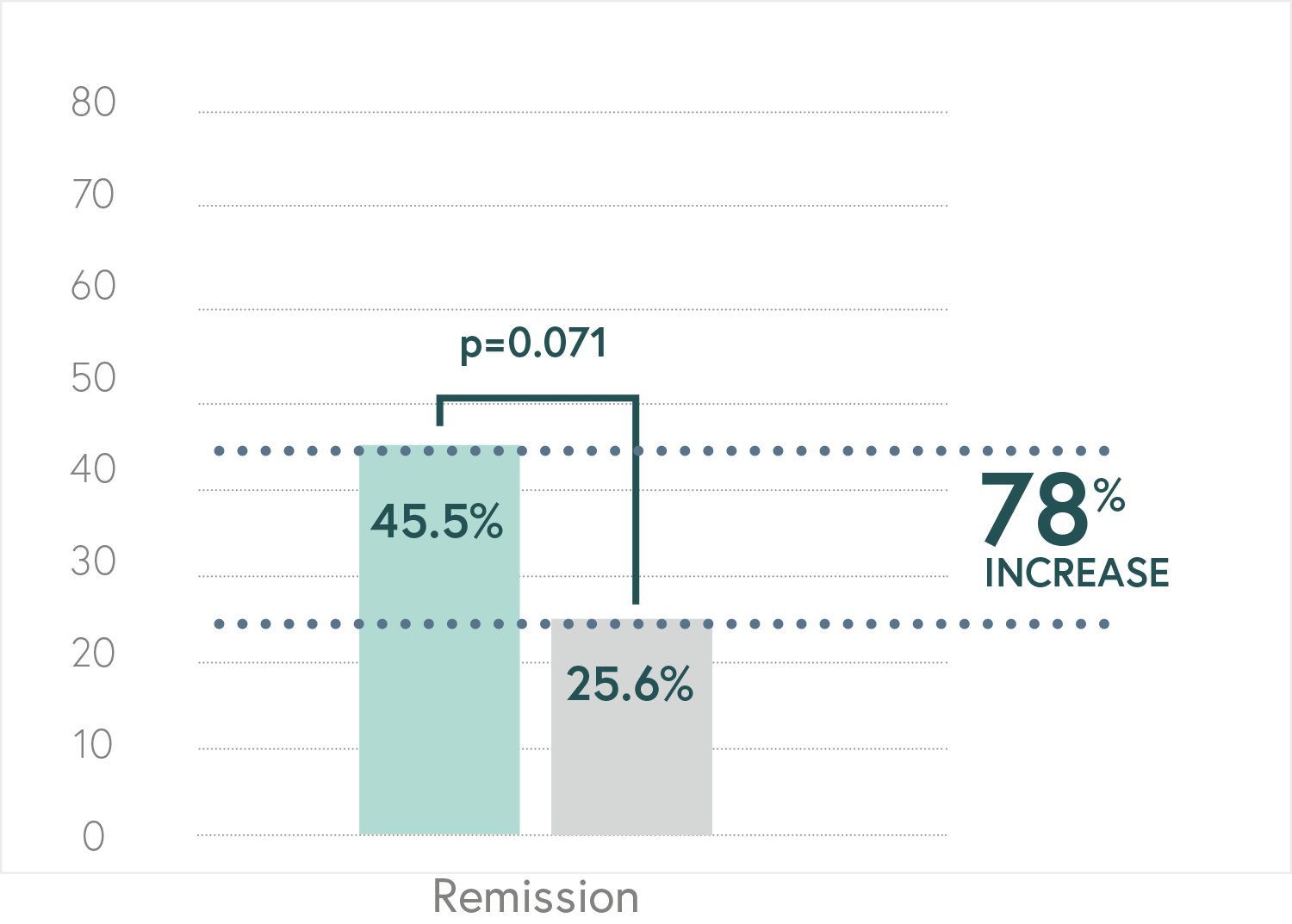
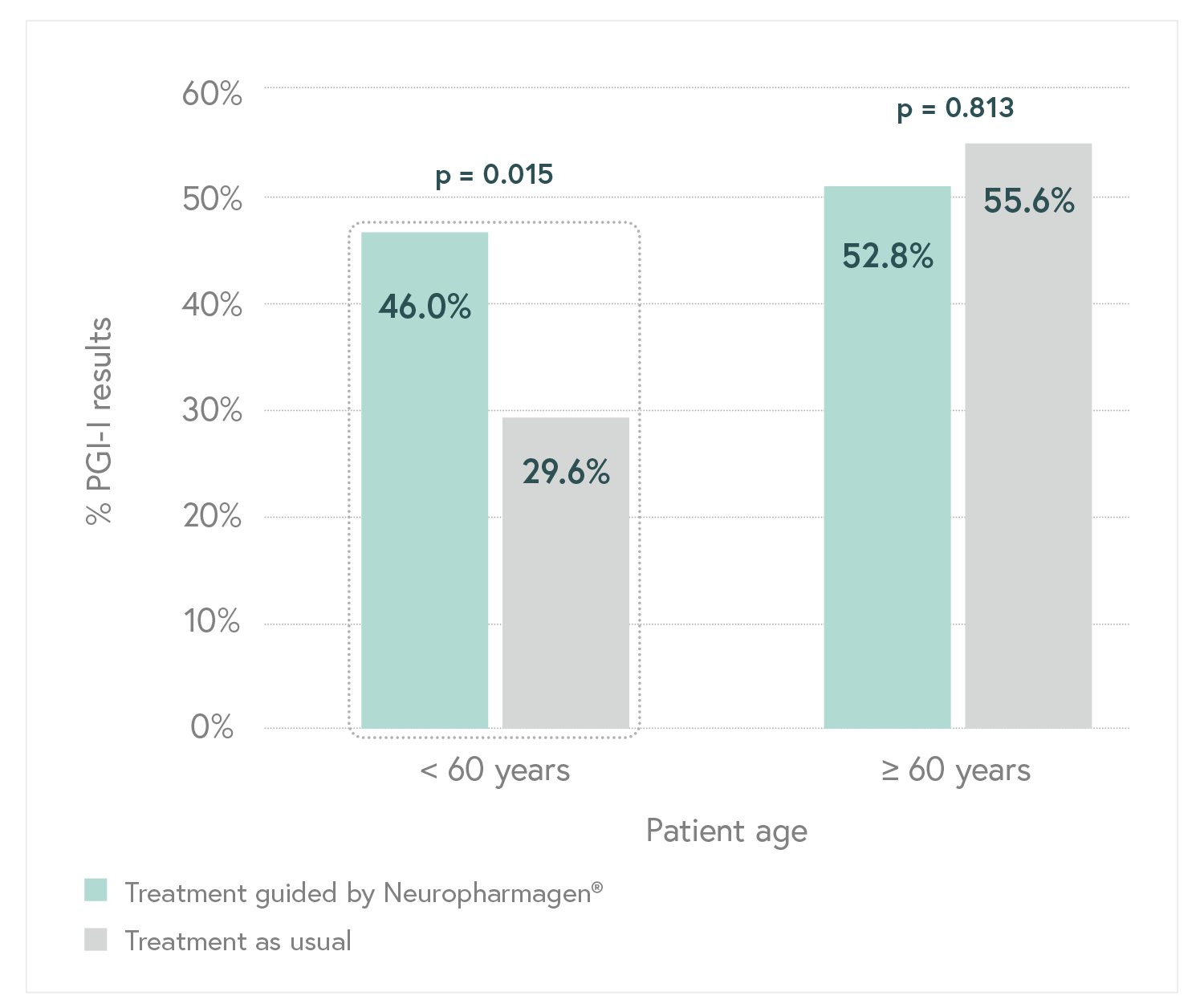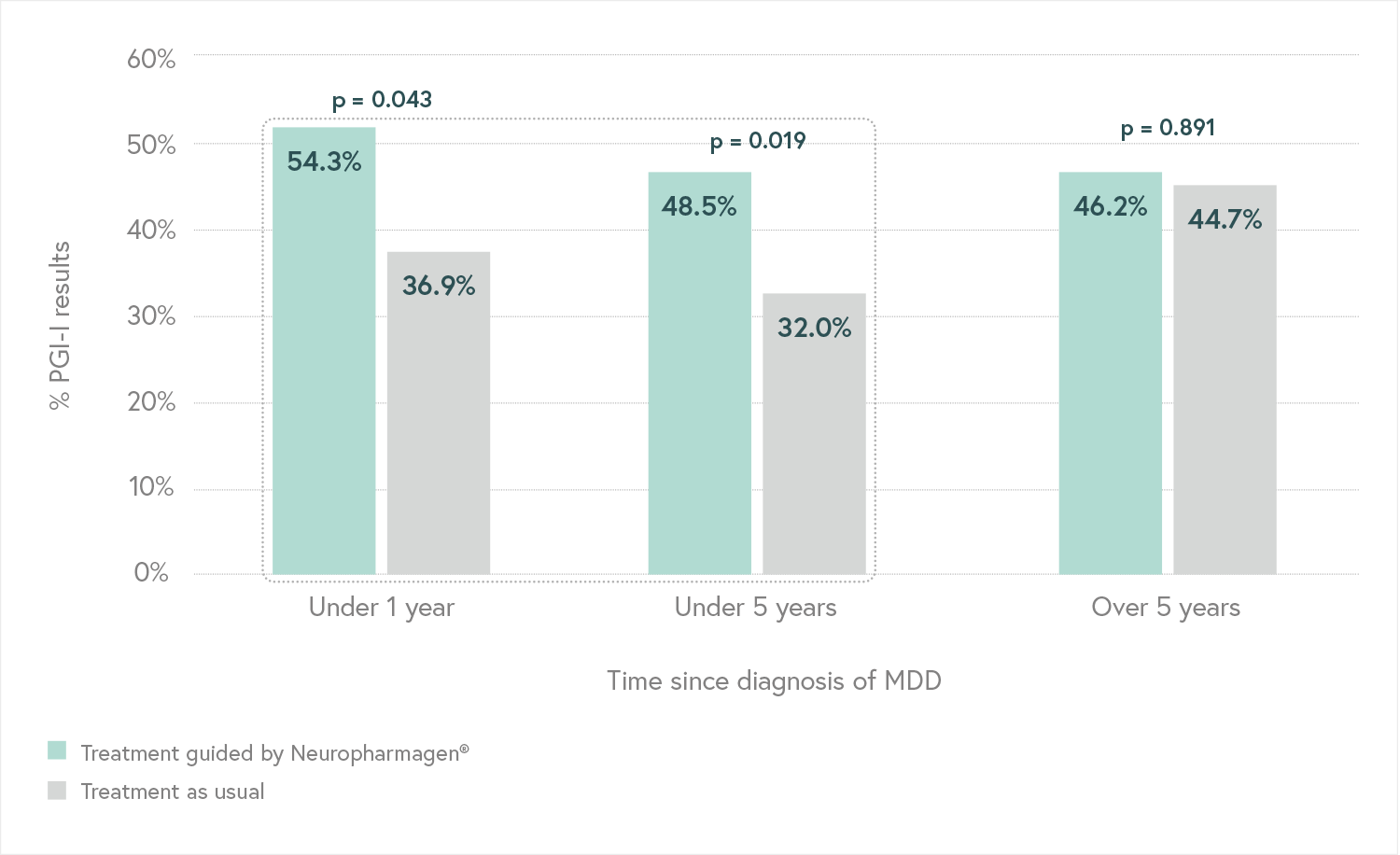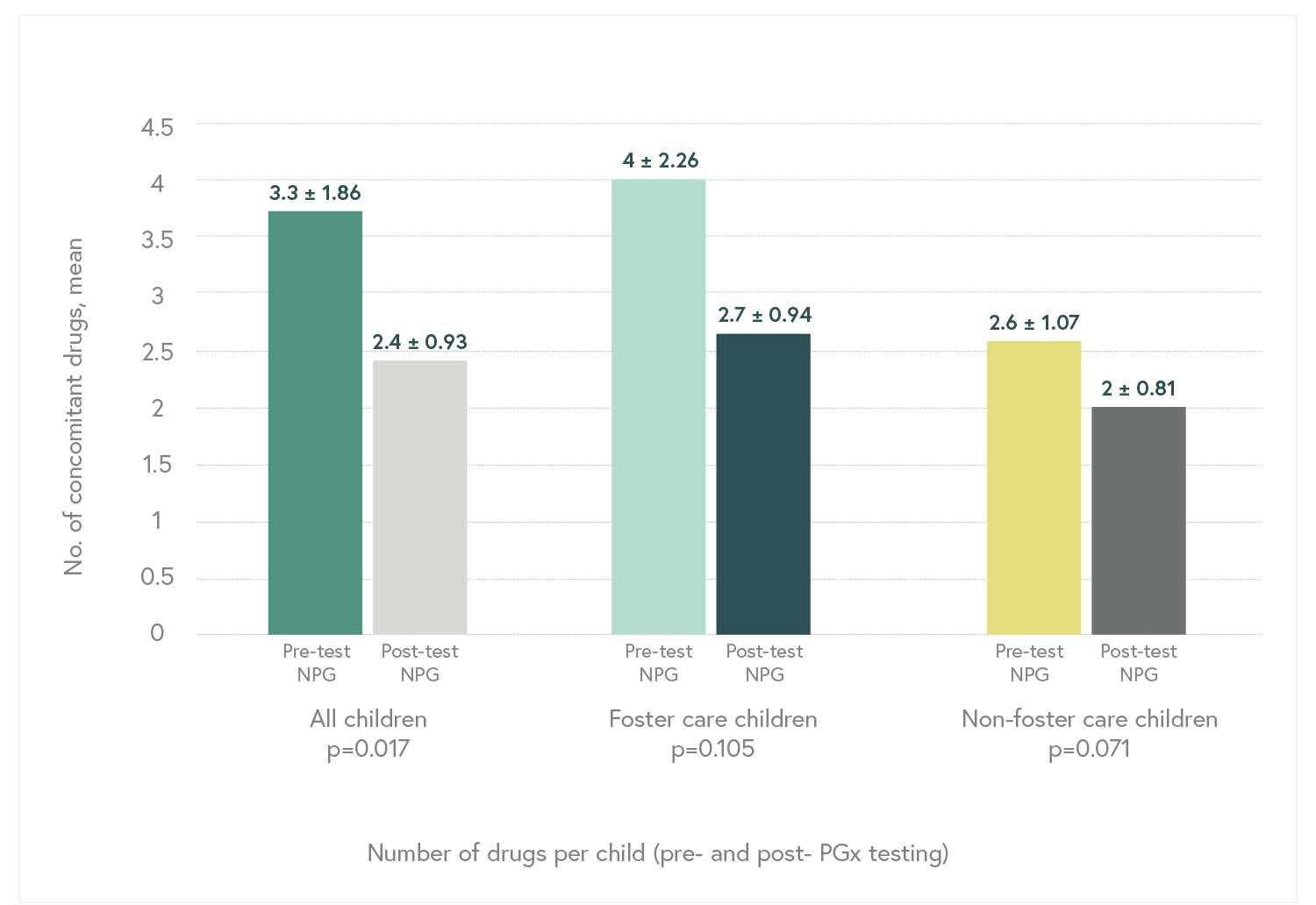Randomized, double-blind, controlled, naturalistic, multicentric, prospective clinical trial that enrolled 316 patients with major depressive disorder
The first large randomized controlled trial (RCT) with a pharmacogenetic-based decision support tool on patients with major depressive disorder, conducted in 18 hospitals in Spain.
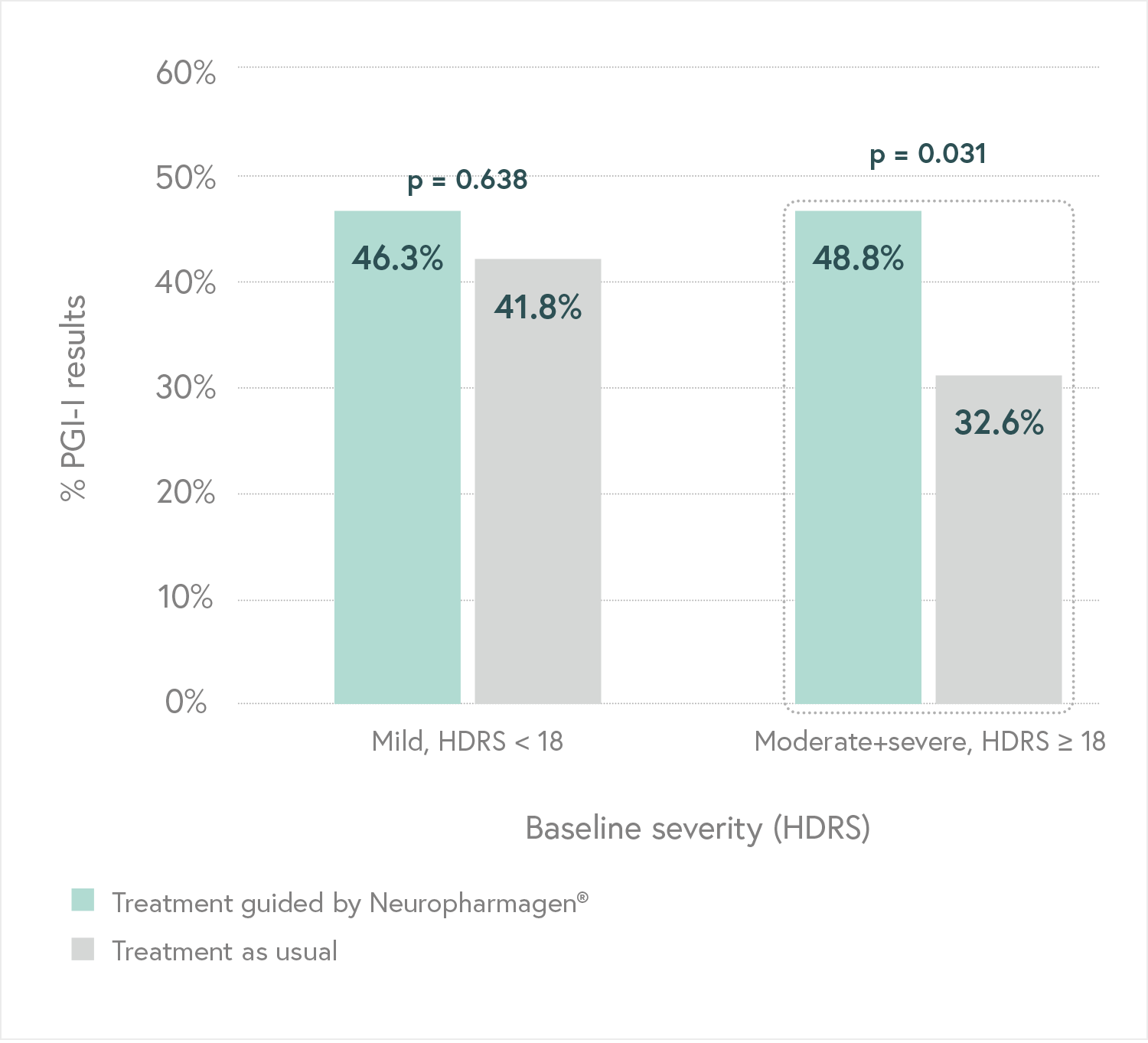
Designed to evaluate the efficacy of Neuropharmagen® in the choice of drug treatment, measured by
the Patient Global Impression of Improvement scale (PGI-I).
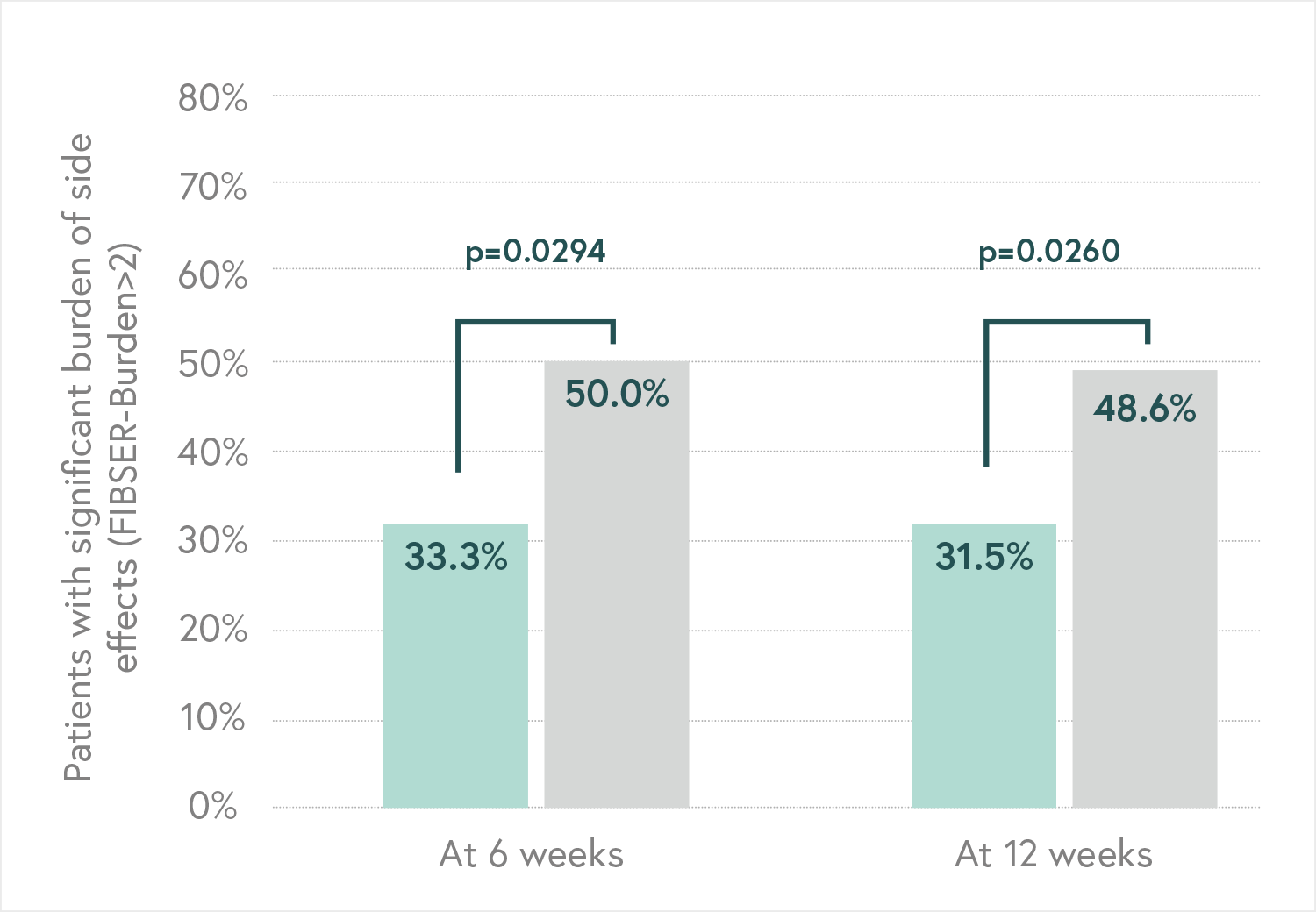
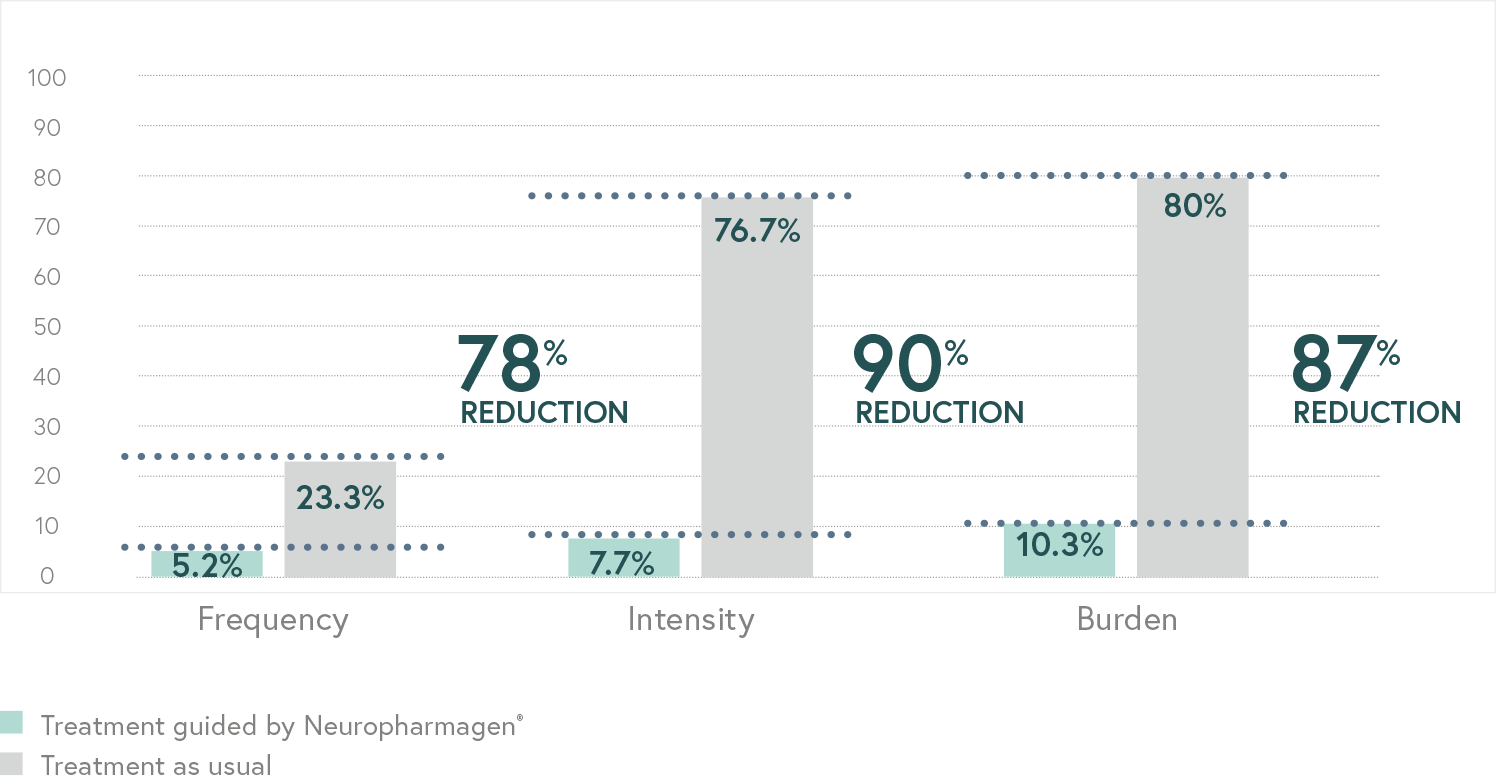
Main Endpoint: The primary variable was the number of patients responding to treatment, which was defined as those who indicated that they felt “Moderately better” or “Much better” on the Patient Global Impression of Improvement scale (PGI-I). Secondary variables such as the Hamilton Depression Scale (HDRS-17) or the FIBSER (Frequency, Intensity, and Burden of Side Effects Ratings) were also analyzed to assess the tolerability of the treatment.